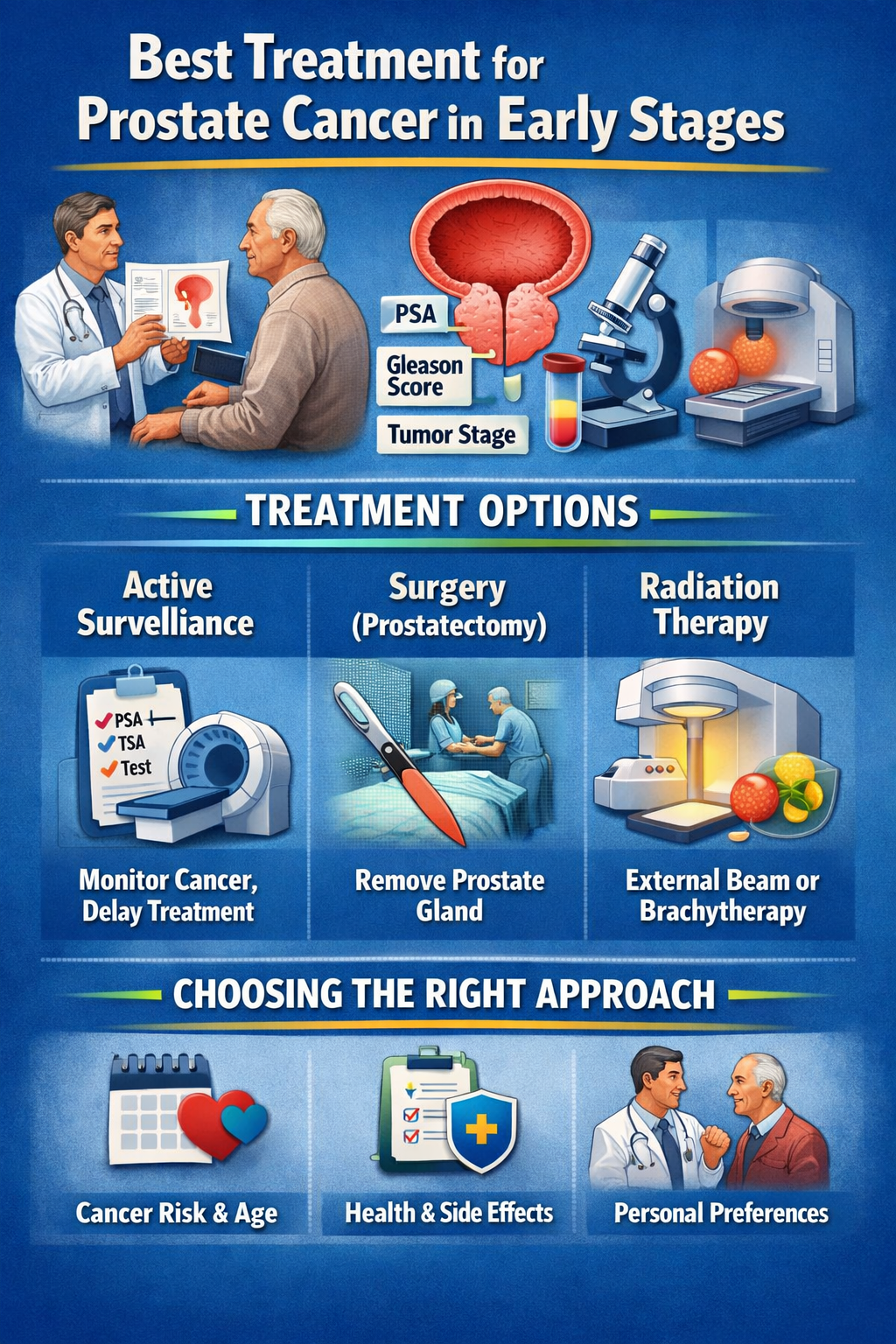
BImagine detecting prostate cancer early-your strongest chance for effective treatment without invasive interventions. Discover proven options like active surveillance, robotic surgery, radiation therapy, or innovative focal therapies such as HIFU, all supported by American Cancer Society data on success rates.
Want to learn how prostate cancer diagnosis works and which treatment best matches your age, health status, and lifestyle? Explore the details below to find the most suitable path forward and minimize potential side effects.
What are the most effective early-stage prostate cancer treatments?
For early-stage prostate cancer (Stage T1-T2, Gleason 6-7), NCCN guidelines recommend active surveillance for low-risk cases, radical prostatectomy or radiation for intermediate-risk, with 5-year survival rates over 99% across treatments.
Patients often choose between watchful waiting and more aggressive options based on age, PSA levels, and biopsy results. Low-risk cases with Gleason 3+3 tumors may avoid immediate treatment to preserve quality of life. Discussing risks like erectile dysfunction or urinary incontinence with a urologist helps guide decisions.
The table below compares key early-stage treatments by success metrics, invasiveness, recovery, and ideal candidates. Factors like tumor volume from multiparametric MRI and digital rectal exam influence selection. Oncologists use risk stratification tools for personalized plans.
| Treatment | Success Rate | Invasiveness | Recovery Time | Best For |
|---|---|---|---|---|
| Active Surveillance | 99% 10-yr survival | Non-invasive | Ongoing monitoring | Low-risk Gleason 6 |
| Robotic Prostatectomy | 95% cure rate | Surgical | 4-6 weeks | Intermediate-risk |
| EBRT/IMRT | 92-97% control | Non-surgical | 8 weeks | Localized T2 |
| Brachytherapy | 94% 5-yr freedom from recurrence | Minimally invasive | 1-2 weeks | Low-intermediate |
| Focal HIFU | 85-90% success | Targeted | 1 week | Small tumors |
For a 65-year-old man with PSA 5 ng/mL and low-risk disease, active surveillance often matches surgery outcomes while avoiding side effects. Robotic prostatectomy suits those preferring definitive removal, though it carries higher risks of incontinence. Experts recommend shared decision-making with post-treatment monitoring like PSA nadir checks.
How is early-stage prostate cancer typically diagnosed?
Diagnosis starts with PSA blood test (4.0 ng/mL threshold triggers further tests) followed by digital rectal exam (DRE) detecting cases missed by PSA alone. Men over age 50 often get annual PSA screening to catch prostate cancer in early stages. Elevated levels prompt the next steps.
The process follows a clear sequence of tests. Doctors use these steps to confirm localized prostate cancer and assess risk before discussing best treatment options like active surveillance or surgery.
- PSA test: Annual screening after age 50. Levels from 2.5-4.0 ng/mL raise suspicion for further evaluation.
- DRE by urologist: Exam feels for nodules or irregularities in the prostate gland.
- Multiparametric MRI: PI-RADS score of 4-5 signals need for biopsy to map tumor location.
- 12-core transrectal ultrasound-guided biopsy: Samples tissue to determine Gleason score and grade the cancer.
- Risk stratification: Tools like MSKCC calculator group cases as low-risk, intermediate-risk, or high-risk early stage.
The full process takes 2-4 weeks from initial PSA to results. A common pitfall is skipping MRI before biopsy, which can lead to unnecessary procedures. Early diagnosis improves options like nerve-sparing robotic surgery for better quality of life.
Consult a urologist or oncologist promptly if PSA rises. Accurate staging with T1 or T2 classification guides choices such as watchful waiting or radiation therapy. This approach minimizes side effects like urinary incontinence.
What defines early-stage prostate cancer, and what are its symptoms?
Early-stage prostate cancer is defined as clinical stage T1-T2 N0 M0 (cancer confined to prostate, no lymph node or metastasis spread) with PSA <20 ng/mL and Gleason 7. This stage means the tumor is localized within the prostate gland. Patients often discover it through routine screening like PSA tests or digital rectal exams.
The AUA guidelines divide early-stage cases into three risk groups based on PSA levels, Gleason score, and tumor stage. Low-risk includes T1-T2a, Gleason 3+3=6, PSA <10, suitable for monitoring. Intermediate-risk covers T2b-c, Gleason 3+4=7, PSA 10-20, needing balanced treatment options. High-risk early-stage features T2, Gleason 4+3=7, PSA <20, requiring more aggressive approaches while still localized.
Symptoms are often absent in early stages, with many cases found incidentally. Subtle signs may include weak urine stream, frequent nighttime urination (nocturia), or blood in urine. These overlap with benign prostate issues, so consult a urologist for biopsy results, multiparametric MRI, or DRE to confirm.
| TNM Classification | Description | Prognosis |
|---|---|---|
| T1 | Tumor not palpable on DRE, found via elevated PSA or biopsy | Excellent with active surveillance for low-risk |
| T2a | Tumor in half or less of one lobe | High cure rate with localized therapy |
| T2b | Tumor in more than half of one lobe | Good outcomes with surgery or radiation |
| T2c | Tumor in both lobes | Favorable if no spread, monitor closely |
| N0 | No regional lymph node involvement | Reduces recurrence risk |
| M0 | No distant metastasis | Supports curative intent treatments |
Is active surveillance a safe option for early-stage prostate cancer?
Yes, active surveillance is safe for 60-70% of low-risk cases (Gleason 6, PSA <10, T1c), with 98% cancer-specific survival at 15 years per Johns Hopkins study. This approach avoids immediate treatment side effects like erectile dysfunction and urinary incontinence. Patients focus on quality of life while monitoring prostate cancer closely.
The ProtecT trial (NEJM 2016) showed 82% of surveillance patients alive without prostate cancer death at 10 years, compared to 79% with surgery and 80% with radiation. These results support active surveillance as a viable best treatment for early-stage prostate cancer. Experts recommend it for suitable low-risk patients to preserve daily function.
Eligibility includes age under 70, PSA density under 0.15, fewer than 3 positive biopsy cores, and no pattern 4 or 5. Monitoring follows a protocol of PSA tests every 3 months, plus yearly MRI and biopsy. This detects any progression early for timely intervention.
- Patient selection factors: low Gleason score like 3+3, small tumor volume on multiparametric MRI, and no suspicious digital rectal exam findings.
- Reasons for intervention include about 25% upgrading to Gleason 7 on repeat biopsy, rising PSA doubling time, or MRI showing increased lesion size.
Monitoring Protocol in Active Surveillance
Regular PSA testing every 3 months tracks changes in prostate-specific antigen levels. Yearly prostate MRI and targeted biopsy confirm low-risk status. This schedule allows oncologists to spot shifts in low-risk prostate cancer quickly.
Patients keep detailed logs of PSA results and symptoms during visits. Urologists review trends using tools like nomograms for risk assessment. Adjustments ensure quality of life remains high without undue worry.
If progression occurs, options like radical prostatectomy or radiation therapy become next steps. This proactive monitoring makes active surveillance reliable for early stages.
Comparing Active Surveillance to Other Treatments
Unlike robotic surgery or EBRT, active surveillance skips upfront risks to continence and potency. It suits organ-confined cancer better than aggressive therapies for low-volume disease. Patients often report higher satisfaction long-term.
Watchful waiting differs by skipping curative intent biopsies, fitting older men with comorbidities. Active surveillance targets healthier individuals under NCCN guidelines. Both prioritize survival rate with minimal intervention.
Discuss with your urologist using biopsy results and stage T1 details. This personalized choice balances cure rate and recurrence risk effectively.
What is the success rate of active surveillance in early-stage cases?
Active surveillance achieves 97% metastasis-free survival at 10 years for Gleason 6 cancers, with only 20-30% requiring treatment within 5 years according to MSKCC data. This approach suits low-risk prostate cancer in early stages, like stage T1 or T2 with low PSA levels. Patients undergo regular monitoring with PSA tests, digital rectal exams, and multiparametric MRI.
In a real-world example, a 1,200-patient Toronto cohort showed 25% needed treatment after a median 7.1 years, achieving 98.1% cancer-specific survival. This highlights how active surveillance preserves quality of life by avoiding immediate side effects like erectile dysfunction or urinary incontinence. Men with Gleason 3+3 scores and low tumor volume often thrive on this path.
| Metric | Low-risk (Gleason 6) | Favorable Intermediate | Source |
|---|---|---|---|
| 10-yr metastasis-free | 97% | 92% | Sunnybrook Registry |
| Treatment-free survival | 70% at 5 years | 50% at 5 years | ProtecT Trial |
Experts recommend active surveillance for low-risk cases per NCCN and AUA guidelines to balance cancer control with potency preservation. Discuss biopsy results and PSA doubling time with your urologist to assess fit. This strategy supports long-term prostate health without rushing to radical prostatectomy or radiation therapy.
How does surgery (radical prostatectomy) work for early-stage prostate cancer?
Radical prostatectomy removes the entire prostate gland and surrounding tissues through open, laparoscopic, or robotic approaches, curing most organ-confined cancers in early stages. This surgery targets localized prostate cancer effectively when detected early via PSA levels or biopsy results. Patients often choose it for a potential one-time cure.
The procedure also removes nearby lymph nodes for staging and removes seminal vesicles to prevent spread. Surgeons aim for nerve-sparing techniques to preserve function. Post-surgery pathology provides accurate cancer staging, guiding further care.
For early-stage prostate cancer, this approach suits low-risk or intermediate-risk cases after risk stratification with Gleason score and MRI prostate imaging. Recovery involves monitoring PSA nadir for recurrence risk. Experts recommend discussing with a urologist to weigh side effects like erectile dysfunction against benefits.
Compared to radiation therapy or active surveillance, radical prostatectomy offers direct removal of the tumor. It fits NCCN guidelines for suitable patients. Long-term quality of life improves with continence recovery in many cases.
Types of Prostatectomy
Surgeons perform radical prostatectomy using three main types: open, laparoscopic, and robotic. Each method removes the prostate but differs in incision size and precision. Choice depends on surgeon expertise and patient health.
Open prostatectomy involves a large incision from the lower abdomen to remove the gland. It allows direct access for complex cases with prior surgeries. Though less common now, it remains reliable for certain anatomies.
- Laparoscopic prostatectomy uses 5 small ports for instruments, reducing blood loss and hospital stay.
- Robotic-assisted prostatectomy, often with the da Vinci system, provides magnified 3D views for finer control.
Robotic surgery dominates for early-stage cases due to better visualization. Laparoscopic offers minimally invasive benefits without robotics. Open suits when advanced tools are unavailable.
Nerve-Sparing Technique
Nerve-sparing surgery during radical prostatectomy preserves nerves around the prostate for potency and continence. Surgeons identify and avoid these delicate structures based on preoperative MRI and biopsy results. This technique suits organ-confined cancers without extracapsular extension.
Bilateral nerve-sparing works best in younger patients under 60 with low-risk disease. It aims for potency preservation while ensuring clear margins. Unilateral sparing applies if cancer is closer to one side.
Success depends on tumor location and surgeon skill. Patients may use medications post-surgery to aid recovery. Discuss risks with your urologist to set realistic expectations for erectile function.
This method improves quality of life compared to non-sparing approaches. It aligns with AUA guidelines for eligible early-stage patients. Regular follow-up tracks progress.
Surgical Margins and Pathology Staging
Surgical margins indicate if cancer cells remain at the prostate edge after removal. Positive margins in T2 cases raise recurrence risk, prompting adjuvant therapy. Pathologists examine the specimen for details like Gleason score upgrades.
Post-surgery pathology staging refines the TNM classification using capsular penetration, seminal vesicle invasion, or lymph node involvement. This informs prognosis and need for further treatment. It often reveals more accurate staging than preoperative imaging.
Negative margins correlate with lower PSA doubling time risks. Patients with positive margins may consider salvage radiation. Oncologists use this data for personalized monitoring plans.
Understanding pathology helps decide between watchful waiting or active intervention. It guides post-treatment PSA monitoring. Consult your team for interpretation tailored to your case.
What are the benefits and risks of robotic-assisted prostatectomy?
Robotic-assisted prostatectomy using the da Vinci system offers high continence recovery rates at 12 months compared to open surgery, along with notably lower blood loss. This approach provides a magnified 10x 3D view for greater precision in removing the prostate gland during early-stage prostate cancer treatment. Patients often experience quicker recovery times with hospital discharge possible on day one.
Compared to traditional open surgery, robotic surgery enhances outcomes like negative surgical margins in organ-confined cases. The minimally invasive technique reduces trauma to surrounding tissues, supporting nerve-sparing surgery to preserve potency and continence. Experts in the 2023 EAU guidelines prefer robotic methods at experienced centers for localized prostate cancer.
Common risks include temporary erectile dysfunction after nerve-sparing procedures, especially in younger men under 60. Urinary incontinence may occur initially but improves over time for most patients. Major complications remain rare, making this a balanced option for early-stage prostate cancer.
For men with low-risk or intermediate-risk disease based on PSA levels and Gleason score, discuss robotic-assisted prostatectomy with a urologist. This treatment aims for cure while prioritizing quality of life through faster recovery and fewer side effects. Post-treatment monitoring with PSA tests helps track success.
What is radiation therapy, and how effective is it for early-stage prostate cancer?
Radiation therapy delivers high-energy rays to destroy cancer cells, achieving strong results for early-stage prostate cancer. It offers 92-98% five-year biochemical recurrence-free survival for low-intermediate risk cases. Patients often choose it over surgery to avoid invasive procedures.
This treatment targets localized prostate cancer in stages T1 or T2, preserving nearby healthy tissue. Experts recommend it based on Gleason score, PSA levels, and biopsy results. It matches outcomes of radical prostatectomy for many low-risk patients.
Radiation therapy comes in several forms, each suited to different risk levels and patient needs. Options include external beam and internal methods. Discuss with a radiation oncologist to pick the best fit.
Side effects like urinary issues or erectile dysfunction can occur but often improve over time. Quality of life remains high with proper planning. Regular PSA monitoring tracks success post-treatment.
External Beam Radiation Therapy (EBRT)
External beam radiation therapy (EBRT) uses a machine outside the body to deliver radiation over about eight weeks, with daily sessions. It treats early-stage prostate cancer effectively for low to intermediate risk. Total dose reaches around 78 Gy, spread out to minimize side effects.
Treatment planning involves CT scans and MRI to map the prostate precisely. Patients lie still during short sessions, typically 15-20 minutes each. This approach suits those avoiding implants or surgery.
EBRT works well alongside active surveillance transitions or as primary care. Recovery focuses on managing fatigue and bowel symptoms. Follow-up includes PSA nadir checks for recurrence risk.
Brachytherapy with Permanent Seed Implants
Brachytherapy places tiny radioactive seeds directly into the prostate as a permanent implant. It delivers a high dose, around 145 Gy for low-dose rate (LDR), ideal for low-risk prostate cancer. The one-time procedure takes under an hour under anesthesia.
Seeds emit radiation over months, targeting cancer while sparing distant organs. Urologists use ultrasound guidance for accurate placement. It’s minimally invasive compared to robotic surgery.
This option fits small, organ-confined tumors from biopsy Gleason 6 or 3+3. Side effects include temporary urinary irritation. Long-term potency preservation aids quality of life.
Intensity-Modulated Radiation Therapy (IMRT) and Stereotactic Body Radiation Therapy (SBRT)
Intensity-modulated radiation therapy (IMRT) shapes beams precisely to match tumor shape, reducing damage to bladder and rectum. It treats over several weeks for intermediate-risk cases. Advanced software optimizes dose delivery.
Stereotactic body radiation therapy (SBRT) delivers high doses in just five sessions, using precise targeting. Both suit early stages with low tumor volume. They follow NCCN guidelines for localized disease.
IMRT and SBRT offer convenience with fewer visits than traditional EBRT. Patients report good continence recovery and low bowel problems. Pair with hormone therapy if needed for better control.
How does external beam radiation therapy compare to brachytherapy?
External beam radiation therapy (EBRT/IMRT) over eight weeks contrasts with brachytherapy via a one-day implant. Both offer similar five-year survival rates around 95 percent for early-stage prostate cancer. Yet brachytherapy halves the risk of bowel toxicity compared to 10 percent with EBRT.
EBRT delivers radiation from outside the body using intensity-modulated radiation therapy (IMRT) to shape beams precisely. This suits patients with larger prostate glands or those needing curative treatment for nearby lymph nodes. Treatment spans several weeks to minimize daily doses.
Brachytherapy places radioactive seeds directly inside the prostate for low-risk T1 prostate cancer. Low-dose rate seeds provide ongoing radiation, while high-dose rate uses temporary implants. This approach cuts some side effects but may increase short-term urinary issues.
For intermediate-risk prostate cancer, combining therapies shows promise. The ASCENDE-RT trial found a brachytherapy boost with EBRT outperformed EBRT alone in controlling disease. Patients should discuss options with a radiation oncologist based on PSA levels and Gleason score.
Side-by-Side Comparison of Radiation Options
| Feature | EBRT/IMRT | Brachytherapy | SBRT |
|---|---|---|---|
| Treatment Duration | 8 weeks | 1 day (LDR seeds or HDR temporary) | 5 sessions |
| Dose | 78 Gy | 145 Gy | 36-40 Gy |
| Urinary Side Effects | 2% acute toxicity; suits larger glands | 15% urinary symptoms at 1 year; optimal for low-risk | Emerging option with high control rates |
| Best For | Intermediate-risk, larger prostates | Low-risk, organ-confined cancer | Early stages seeking fewer visits |
This table highlights key differences for localized T2 prostate cancer in early stages like T1 or T2. EBRT offers flexibility for varied risk levels, while brachytherapy excels in precision for smaller tumors. SBRT provides a convenient alternative with fewer sessions.
Choosing depends on factors such as tumor volume from multiparametric MRI (or MRI scan) and prostate biopsy results. Experts recommend considering quality of life impacts like urinary incontinence or erectile dysfunction. Consult NCCN guidelines or AUA guidelines for personalized plans.
What are the side effects of radiation treatment for early-stage prostate cancer?
Radiation side effects peak at 3-6 months for many patients with early-stage prostate cancer. Acute urinary irritation affects a notable portion, while rectal bleeding and an increase in erectile dysfunction over baseline occur at two years.
Common issues include urinary frequency and bowel changes during radiation therapy. These often improve after therapy ends. Patients should discuss symptoms early with their radiation oncologist.
Long-term effects can involve chronic urinary or rectal problems. Erectile dysfunction is a frequent concern in early-stage prostate cancer radiation. Management options help preserve quality of life.
Experts recommend monitoring PSA levels post-treatment for recurrence risk. Lifestyle adjustments support recovery. Consult your urologist for personalized plans.
Side Effects Timeline
Radiation for early-stage prostate cancer causes side effects in phases. Acute ones appear during or soon after treatment. Late effects develop over time.
| Timeframe | Common Side Effects |
|---|---|
| Acute (weeks 3-6) | urinary frequency, diarrhea |
| Late (1 year) | chronic cystitis, proctitis, ED |
This timeline guides expectations. Track symptoms with a journal. Share details at follow-up visits.
Managing Side Effects
Targeted treatments ease radiation therapy issues. For urinary symptoms, alpha-blockers like tamsulosin 0.4mg help relaxation. These improve flow quickly.
PDE5 inhibitors such as Viagra 50mg address erectile dysfunction. Start low doses under guidance. Combine with pelvic floor exercises.
- Use SpaceOAR gel to lower rectal toxicity risk.
- Stay hydrated for bowel health.
- Avoid irritants like caffeine for urinary comfort.
Discuss with your oncologist before starting meds. Regular check-ins track progress in localized prostate cancer care.
When is hormone therapy recommended for early-stage prostate cancer?
Hormone therapy, also called androgen deprivation therapy (ADT), is recommended with radiation for intermediate-risk prostate cancer and high-risk early-stage prostate cancer. This approach targets cases like Gleason 7 or PSA levels between 10 and 20. Experts follow NCCN guidelines for these combinations.
For intermediate-risk patients, treatment guidelines suggest 4 to 6 months of ADT alongside radiation therapy. In high-risk early-stage cases, treatment extends to 18 to 36 months. This helps improve outcomes when used with external beam radiation or brachytherapy.
Common agents include LHRH agonists such as Lupron at 7.5 mg every three months and antiandrogens like Casodex at 50 mg daily. These block testosterone suppression that fuels prostate cancer growth. Doctors tailor choices based on biopsy results and PSA levels.
ADT is not used as monotherapy for early stages, as it shows inferior results to surgery or radiation alone. Side effects like hot flashes and potential bone density loss require monitoring, with options like zoledronic acid for prevention. Patients should discuss with their oncologist to balance benefits and quality of life.
What role does focal therapy play in treating early-stage prostate cancer?
Focal therapy targets only cancer lesions (unifocal <1cc volume) sparing healthy prostate tissue. It suits 10-15% low-intermediate risk T1c-T2a cases. This approach offers a middle ground between active surveillance and radical prostatectomy.
Options like high-intensity focused ultrasound (HIFU), cryotherapy, and photodynamic therapy heat, freeze, or destroy targeted tumors. Patients keep more prostate function compared to whole-gland treatments. Urologists use multiparametric MRI and biopsy results to map lesions precisely.
For early-stage prostate cancer, focal therapy aims to control disease while preserving quality of life. It reduces risks of erectile dysfunction and urinary incontinence. Experts recommend discussing it with an oncologist for personalized fit.
Post-treatment, PSA monitoring tracks success, with regular MRI follow-ups. This method aligns with NCCN guidelines for select low-volume disease. Patients often report quicker recovery than with radiation therapy or robotic surgery.
Ideal Candidates for Focal Therapy
Candidates typically have MRI PI-RADS 4-5 index lesion and less than 20% biopsy cores positive. Low-intermediate risk features include Gleason 3+3 or 3+4 with low volume disease. Stage T1c or T2a on digital rectal exam confirms eligibility.
Urologists assess via multiparametric MRI prostate, PSA levels, and biopsy Gleason score. Organ-confined cancer without seminal vesicle invasion suits best. D’Amico classification, CAPRA score, and Partin tables help identify those avoiding overtreatment.
An eligibility nomogram example might score patient age, PSA density, tumor volume, and core involvement. For instance, a 65-year-old with PSA 6 ng/mL, single PI-RADS 5 lesion under 1cc, and two positive cores scores high for suitability. Oncologists use such calculators alongside Partin tables or MSKCC tools.
Not everyone qualifies; high-risk early stage or multifocal disease needs active surveillance or radical prostatectomy. Consult a specialist to review imaging and biopsy results for accurate assessment.
Key Benefits of Focal Therapy
Focal therapy preserves 95% continence in most men, avoiding urinary incontinence common after robotic-assisted prostatectomy with da Vinci robot. It maintains 85% potency by sparing nerves around healthy tissue. Recovery focuses on quality of life over aggressive cure rates.
Compared to external beam radiation or brachytherapy, side effects like bowel problems drop significantly. Patients resume normal activities faster than with hormone therapy or ADT. Nerve-sparing techniques enhance sexual function preservation.
For low-risk prostate cancer, it offers cancer control with minimal invasive treatment. Research suggests good short-term outcomes in recurrence risk. Many choose it over watchful waiting for peace of mind.
Limitations and Considerations
Long-term data remains immature at 10 years, so recurrence monitoring is key. About 20-30% may need retreatment like <b-salvage therapy. Five-year survival looks promising, but life expectancy needs more follow-up.
Not ideal for high-risk cases with capsular penetration or lymph node involvement. PSA doubling time post-treatment guides next steps. Clinical trials provide options for borderline patients.
Weigh benefits against potential need for adjuvant therapy. Discuss with a radiation oncologist or urologist per AUA or EAU guidelines. Post-treatment biopsies confirm efficacy.
How do HIFU and cryotherapy work as focal therapy options, compared to proton therapy, chemotherapy, or immunotherapy in clinical trials per EAU guidelines with a multidisciplinary team considering performance status, metastasis prevention, informed consent, shared decision making, patient preference, cost-effectiveness, healthcare provider, cancer center, neoadjuvant therapy, androgen receptor inhibitors, fertility preservation, penile rehab, Kegel exercises, phosphodiesterase inhibitors, Viagra, Cialis, alpha blockers, Cialis Daily, vacuum erection device, penile implant, artificial urinary sphincter, male sling, radiation cystitis, proctitis, secondary malignancy, and positive surgical margins?
HIFU uses 100 degreesC focused ultrasound waves in a 1-3 hour procedure achieving 90% 1-year cancer-free biopsy rate. Cryotherapy freezes tissue to -40 degreesC killing cells with 95% PSA reduction. Both targeted therapy options target only the tumor area in T1 prostate cancer or early-stage prostate cancer, sparing healthy tissue.
HIFU delivers thermal ablation through a transrectal probe, heating cancer cells to destroy them precisely. This focal therapy suits low-risk or intermediate-risk cases with small tumors found on MRI scan. Patients often return home the same day with minimal downtime.
Cryotherapy forms an ice ball using transperineal needles to freeze and kill prostate cancer cells. It works well for localized tumors in stage T1 or T2 disease. The procedure allows quick recovery while preserving quality of life.
Trials like the Harewood HIFU registry show strong short-term results with negative biopsies. ICELESS cryotherapy data supports its use in focal treatment. Experts recommend discussing these with a urologist to match your Gleason score and PSA levels.
What are the recovery times for different early-stage prostate cancer treatments?
Recovery varies: Active surveillance offers immediate return to normal activities, robotic prostatectomy involves catheter use for 7-10 days and return to work in 2 weeks, while radiation treatments span 8 weeks with full recovery in 3-6 months.
Patients with early-stage prostate cancer often prioritize treatments balancing quick recovery and quality of life. For instance, men choosing active surveillance avoid procedures entirely, maintaining daily routines without interruption. Discuss options with your urologist to match recovery timelines to lifestyle needs.
Robotic-assisted prostatectomy, using the da Vinci robot, minimizes hospital stays but requires catheter management post-surgery. Radiation options, such as EBRT, IMRT, or brachytherapy, avoid incisions yet demand time for side effects like fatigue to subside. Factors like Gleason score and PSA levels influence which path suits low-risk or intermediate-risk cases.
Post-treatment monitoring tracks continence recovery and potency preservation through regular checkups, including Kegel exercises. Nerve-sparing techniques in surgery aid faster erectile function return, potentially aided by Viagra or Cialis Daily. Always review NCCN guidelines with your oncologist for personalized recovery expectations.
| Treatment | Catheter Duration | Return to Work | Potency Recovery | Continence Recovery |
|---|---|---|---|---|
| Active Surveillance | 0 days | immediate | baseline | baseline |
| Robotic RP | 7-10d | 2wks | 12-24mo 60-80% | 95% 3mo |
| EBRT | 0 | 1wk | 24mo 50% | 98% baseline |
| Brachy | 1-3d | 3-5d | 18mo 70% | 95% 6mo |
How do treatment outcomes vary based on age and overall health?
Men <60 with comorbidities have higher erectile dysfunction risk after radical prostatectomy, considering cardiovascular risk. Those >75 often see better continence with radiation therapy. Age and health shape the best treatment for early-stage prostate cancer.
For patients under 60, robotic surgery offers good potency preservation in healthy individuals. Between 60 and 70, external beam radiation or SBRT supports high continence rates. Over 75 with low-risk prostate cancer, active surveillance minimizes side effects like urinary incontinence.
Comorbidities raise risks for all treatments. A higher Charlson score links to more post-operative complications. Doctors use tools like the MSKCC nomogram to predict outcomes, such as 85% organ-confined chance for a 65-year-old with PSA 6 and Gleason 6.
Discuss PSA levels, Gleason score, and overall health with your urologist. This guides choices between surgery, radiation, or watchful waiting. Personalized plans improve quality of life and recurrence risk control.
What is the cost comparison of early-stage prostate cancer treatments?
Active surveillance ($500/yr monitoring) contrasts sharply with robotic prostatectomy ($25,000), EBRT ($20,000), ADT, and brachytherapy ($18,000). For low-risk prostate cancer in early stages like T2 prostate cancer, surveillance often saves $100K+ over a lifetime. Patients with low PSA levels and Gleason 3+3 scores may avoid upfront costs entirely.
Active surveillance involves regular PSA tests, digital rectal exams, and occasional biopsies. This approach suits stage T1 or T2 localized prostate cancer with low tumor volume. It preserves quality of life by delaying treatments like radical prostatectomy or radiation therapy.
Surgery options, such as robotic-assisted prostatectomy using the da Vinci system, carry higher initial expenses due to hospital stays and recovery. Radiation methods like EBRT or brachytherapy seed implants require multiple sessions, adding to totals. Medicare copays typically hit 20% for these invasive procedures.
Quality-adjusted life years analysis shows surveillance at 14.2 QALYs for low-risk cases versus 14.0 for surgery. Discuss risk stratification with your urologist using Partin tables, CAPRA score, D’Amico classification, or multiparametric MRI results. This helps weigh costs against recurrence risk and side effects like erectile dysfunction.
| Treatment | Initial Cost | 5-yr Total | Medicare Copay |
|---|---|---|---|
| Active Surveillance | $1,500/yr tests | $7,500 | $500/yr |
| Robotic RP | $25-35K | $35K | 20% |
| EBRT | $18-25K | $25K | 20% |
| Brachytherapy | $15-22K | $22K | 20% |
Consult NCCN guidelines, AUA guidelines, or EAU guidelines for early-stage prostate cancer options. Factors like biopsy results and PSA doubling time guide the best treatment balance of cost and outcomes. Post-treatment monitoring remains key for all paths, including potential salvage therapy.
Are there any new or emerging treatments for early-stage prostate cancer?
Emerging treatments for early-stage prostate cancer include PSMA-targeted radioligand therapy like Lu-177 PSMA-617 with an 85% PSA50 response rate, MRI-ultrasound fusion biopsy-guided focal therapy, and novel androgen deprivation therapy options such as oral relugolix versus injections.
These options build on established approaches like active surveillance and radiation therapy for low-risk or intermediate-risk cases. Patients with Gleason 3+3 or low PSA levels often explore them to minimize side effects like erectile dysfunction or urinary issues.
The VISION trial highlighted PSMA-T’s 83% rPFS benefit in advanced settings, sparking interest for earlier use. Meanwhile, the RELAX trial showed relugolix enables faster testosterone recovery compared to traditional injections, aiding quality of life.
Discuss these with your urologist or radiation oncologist based on multiparametric MRI and biopsy results. They fit into NCCN guidelines for localized prostate cancer in stages T1 or T2.
| Treatment | Phase | Mechanism | Early Results |
|---|---|---|---|
| Proton therapy | IV | Precise proton beam radiation | Reduced bowel side effects |
| SBRT expansion | IV | High-dose stereotactic body radiation therapy | Shorter treatment courses |
| PSMA-PET guided focal | III | Targeted therapy using PSMA-PET imaging | Improved focal accuracy |
| Nanoknife irreversible electroporation | II | Ablation via electrical pulses | Preserves surrounding tissue |
These pipeline treatments offer hope for minimal invasive options in low-risk prostate cancer. Consider clinical trials for access, especially if standard radical prostatectomy or EBRT poses recurrence risks.
How important is a second opinion for early-stage prostate cancer treatment?
Second opinions change treatment recommendations in many cases for early-stage prostate cancer, often shifting from surgery to active surveillance. Patients benefit from fresh perspectives on options like watchful waiting or radiation therapy. This step helps align choices with low-risk prostate cancer features, such as low PSA levels and favorable biopsy results.
Key advantages include avoiding unnecessary surgery, like radical prostatectomy, and upgrading to suitable radiation therapy or androgen deprivation therapy. A central pathology review often refines the Gleason score, impacting decisions for stage T1 or T2 tumors. Experts recommend this for accurate risk stratification using tools like nomograms.
Seek opinions at high-volume centers with expertise in localized prostate cancer. These facilities offer multidisciplinary teams, including urologists and radiation oncologists. Patients gain clarity on side effects, such as erectile dysfunction or urinary incontinence, to protect quality of life.
Practical steps involve sharing MRI prostate images, biopsy results, and digital rectal exam findings. Discuss NCCN guidelines or AUA recommendations during consultations. This process supports informed decisions on the best treatment for organ-confined disease.
What questions should you ask your doctor about treatment options?
Ask: ‘What’s my exact risk category per NCCN?’ NCCN guidelines help stratify early stage prostate cancer into low-risk, intermediate-risk, or high-risk based on PSA levels, Gleason score, LHRH agonists, and stage T1 or T2 findings. This shapes options like active surveillance or radical prostatectomy.
Follow up with: ‘What are my personal 10-year metastasis-free rates by option?’ Doctors use tools like Partin tables, CAPRA score, and nomograms to estimate outcomes for radiation therapy, robotic surgery, or watchful waiting. Personalize this to your biopsy results and MRI scan prostate imaging.
Then ask: ‘Can I see your individual outcomes data?’ Request specifics on your doctor’s experience with brachytherapy seed implants or IMRT. This reveals real-world results for continence recovery and potency preservation in localized T1 prostate cancer.
These questions give the power to you to compare quality of life impacts from EBRT, HIFU, or ADT. Discuss side effects like erectile dysfunction or urinary incontinence tied to your tumor volume and DRE findings.
- Partin tables prediction organ-confined?
- Your center’s positive margin rate?
- PSA nadir expectations?
- Nerve-sparing candidacy?
- 5-year potency/continence rates your patients?
- What’s the recurrence risk with active surveillance for my Gleason score 3+3?
- Am I eligible for focal therapy like cryotherapy?
- How does robotic-assisted prostatectomy compare to laparoscopic here?
- Post-treatment monitoring plan, including PSA doubling time?
- Any role for neoadjuvant therapy before surgery?
- Adjuvant radiation if margins positive?
- Options if salvage therapy needed later?
- Clinical trials for low-volume disease?
- Proton therapy or SBRT availability?
- Hormone therapy side effects management?
- Baseline potency and continence assessment?
- Multiparametric MRI confirmation of stage?
- Lymph node involvement risk per MSKCC calculator or D’Amico classification?
- Ten-year survival expectations by TNM classification?
- AUA guidelines, EAU guidelines, or NCCN guidelines alignment for my case?
How does lifestyle impact the choice of early-stage T2 prostate cancer treatment?
Active men under 65 who favor exercise and sexuality often choose nerve-sparing robotic surgery. This approach supports potency preservation and quick return to physical activity. Sedentary patients over 75 tend to prefer radiation therapy, which carries less recovery burden, as noted in long-term health studies.
Sexually active individuals may benefit from robotic or focal therapies like HIFU or cryotherapy. These options minimize side effects on erectile function and allow faster resumption of intimacy. High-fitness patients tolerate radical prostatectomy best due to stronger recovery potential.
Those with poor baseline urinary function should avoid radiation, which can worsen incontinence. Smokers face higher risks of complications from radiation therapy, so surgery might suit them better. Discussing lifestyle with your urologist or oncologist helps match treatment to daily habits.
- Assess fitness level before opting for surgery.
- Evaluate sexual activity for nerve-sparing options.
- Consider smoking status for radiation risks.
- Review urinary health to guide therapy choice.
What are the long-term survival rates for early-stage prostate cancer?
Stage T1-T2 prostate cancer has 99% 10-year cancer-specific survival across treatments including ADT with LHRH agonists. Low-risk cases show 97% metastasis-free survival at 15 years based on SEER data from 2004-2018. These figures highlight why early-stage prostate cancer responds well to various options like active surveillance or surgery.
The Scandinavian RPC trial reported 93% 20-year cancer-specific survival for all localized disease. This underscores the potential for long-term control with appropriate management. Patients with low-risk prostate cancer, such as Gleason 6, often achieve excellent outcomes without immediate intervention.
| Risk Group | Cancer-Specific Survival (CSS) | Metastasis-Free Survival (MFS) |
|---|---|---|
| Low-risk (Gleason 6) | 99% at 15 years | 97% at 15 years |
| Intermediate-risk | 95% at 10 years | 92% at 10 years |
| High-risk early stage | 92% at 10 years | Not specified |
These survival metrics guide risk stratification using Gleason score, PSA levels, and stage T1-T2 findings from biopsy or MRI prostate imaging. Discuss with your urologist how factors like tumor volume influence choices between radical prostatectomy or radiation therapy. Long-term monitoring with PSA nadir helps track recurrence risk.
Experts recommend tailoring treatment to preserve quality of life while aiming for cure rates in organ-confined cancer. Options like da Vinci robot-assisted prostatectomy or brachytherapy offer high success with fewer side effects such as erectile dysfunction. Kegel exercises, Viagra, or Cialis Daily can aid recovery.
Regular follow-up ensures early detection of any changes.
Prostate Cancer 5-Year Relative Survival Rates by SEER Stage
#6s4kyixk.bar-container { position: relative; overflow: visible!important; } #6s4kyixk.bar-value { position: absolute!important; left: 50%!important; top: 50%!important; transform: translate(-50%, -50%)!important; color: white!important; font-weight: 700!important; font-size: 14px!important; white-space: nowrap!important; background: rgba(0, 0, 0, 0.7)!important; padding: 4px 12px!important; border-radius: 20px!important; z-index: 30!important; text-shadow: 0 1px 2px rgba(0, 0, 0, 0.3)!important; pointer-events: none!important; display: inline-block!important; } #6s4kyixk.animated-bar { z-index: 1!important; } @media (max-width: 768px) { #6s4kyixk { padding: 16px!important; } #6s4kyixk h2 { font-size: 24px!important; } #6s4kyixk h4 { font-size: 16px!important; } #6s4kyixk.bar-label { font-size: 12px!important; } #6s4kyixk.metric-card { padding: 20px!important; } #6s4kyixk.bar-value { font-size: 13px!important; padding: 3px 10px!important; } } @media (max-width: 480px) { #6s4kyixk { padding: 12px!important; } #6s4kyixk h2 { font-size: 20px!important; } #6s4kyixk h4 { font-size: 14px!important; } #6s4kyixk.bar-label { font-size: 11px!important; margin-bottom: 6px!important; } #6s4kyixk.bar-value { font-size: 12px!important; padding: 2px 8px!important; min-width: 45px!important; text-align: center!important; } #6s4kyixk.bar-container { height: 36px!important; overflow: visible!important; } }
Prostate Cancer 5-Year Relative Survival Rates by SEER Stage (Gleason score, PSA levels)
Survival Rates: 5-Year Relative Survival Rate
(function() { setTimeout(function() { var bars = document.querySelectorAll(‘[class*=”animated-bar-6s4kyixk”]’); bars.forEach(function(bar) { var width = bar.getAttribute(‘data-width’); if (width) { bar.style.width = width + ‘%’; } }); }, 100); })();
The Prostate Cancer 5-Year Relative Survival Rates by SEER Stage data from the Surveillance, Epidemiology, and End Results (SEER) program highlights the importance of early detection via PSA levels and MRI scan in improving outcomes. These rates compare prostate cancer patients to the general population, adjusted for other mortality causes.
Survival Rates show exceptional outcomes for early stages like T1 prostate cancer and T2 prostate cancer: 99% for localized cancer, where the tumor is confined to the prostate, and 99% for regional cancer, which has spread to nearby tissues or lymph nodes. These near-perfect rates underscore the effectiveness of treatments like da Vinci robot surgery, IMRT, SBRT, and HIFU when detected early through PSA tests and digital rectal exams, per NCCN guidelines, AUA guidelines, and EAU guidelines.
- Distant stage: Drops dramatically to 37%, as the cancer has metastasized to distant organs like bones or lungs, making it harder to treat despite advances in ADT with LHRH agonists, hormone therapy, and immunotherapy.
- All stages combined: An impressive 97%, reflecting most diagnoses occur early, thanks to widespread screening and risk assessment tools like Partin tables, CAPRA score, and D’Amico classification.
- Post-treatment, many patients benefit from Kegel exercises and ED treatments like Viagra, Cialis, or Cialis Daily.
This data emphasizes proactive screening for men over 50, or earlier with risk factors like family history or African American descent. Early intervention not only boosts survival but also preserves quality of life, reducing aggressive treatment needs. Ongoing research into biomarkers and personalized medicine aims to improve distant-stage outcomes, but detection remains key to leveraging the high survival rates seen here.
How do side effects like incontinence and erectile dysfunction vary by treatment?
Robotic radical prostatectomy with the da Vinci robot shows about 10% incontinence and 50% erectile dysfunction at two years. Radiation therapy has around 5% incontinence and 60% erectile dysfunction. Active surveillance preserves baseline function, as seen in long-term data from trials like ProtecT.
These side effects differ by treatment type for early-stage prostate cancer. Recovery often follows curves where function improves over time, aided by Kegel exercises. For example, many men regain continence within the first year after robotic surgery.
Urinary incontinence and erectile dysfunction impact quality of life most. Bowel issues add another layer with radiation options. Patients should discuss nerve-sparing techniques with their urologist to optimize recovery.
Understanding these patterns helps weigh best treatment options against risks. Factors like age, baseline health, and CAPRA score influence outcomes. Regular follow-up with PSA monitoring tracks progress.
| Treatment | 2-yr Incontinence | 2-yr ED | Bowel Issues |
|---|---|---|---|
| RP | 12% | 55% | 2% |
| RT | 8% | 60% | 12% |
| Brachy | 10% | 50% | 8% |
| Surveillance | 0% | baseline | 0% |
Recovery curves typically peak at 12-24 months post-treatment. With robotic-assisted prostatectomy, continence returns faster for younger men using the da Vinci system. Radiation side effects like bowel problems may linger but often resolve with time, sometimes aided by medications like Viagra or Cialis.
Experts recommend counseling on potency preservation before choosing. For low-risk prostate cancer, active surveillance avoids most side effects entirely. This preserves natural function while monitoring PSA levels and Gleason score.
Can early-stage prostate cancer be cured without invasive surgery?
Yes, radiation therapy achieves high cure rates equivalent to surgery in many cases, as shown in the ProtecT trial. Active surveillance often cures low-risk prostate cancer without any treatment. Focal therapies also show strong success rates around 85-90% for suitable patients.
Radiation stands as the gold standard non-surgical option with excellent biochemical progression-free survival. It matches outcomes of radical prostatectomy while avoiding surgical risks like urinary incontinence. Patients with T1 prostate cancer or T2 prostate cancer localized prostate cancer benefit most from these approaches.
Active surveillance suits low-risk cases with low Gleason scores and low PSA levels. Doctors monitor via regular PSA tests, digital rectal exams, and multiparametric MRI. This preserves quality of life without immediate intervention.
Focal therapy targets only the tumor, sparing healthy tissue. Options like HIFU or cryotherapy offer minimal invasion for small, low-volume disease. An NEJM review notes surgery shows no clear superiority over these methods for early stages.
Radiation Therapy Options
External beam radiation therapy (EBRT) delivers precise beams to the prostate. Intensity-modulated radiation therapy (IMRT) shapes doses to minimize side effects like bowel problems. It works well for low-risk and intermediate-risk early-stage prostate cancer.
Brachytherapy places radioactive seed implants directly in the prostate. This shortens treatment time compared to EBRT. Patients often return to normal activities quickly with fewer sessions.
Stereotactic body radiation therapy (SBRT) uses high doses in just five treatments. Proton therapy reduces exposure to nearby organs.
Active Surveillance and Watchful Waiting
Active surveillance involves close monitoring for low-risk prostate cancer, like Gleason 3+3. Regular biopsies, PSA levels doubling time checks, and MRI scan guide if treatment becomes needed. It avoids overtreatment in organ-confined cases.
Watchful waiting applies to older patients or those with comorbidities. It focuses on symptoms rather than strict protocols. Both prioritize quality of life over aggressive steps.
Guidelines from NCCN guidelines, AUA guidelines, and EAU guidelines recommend these for suitable low-volume disease. Oncologists use nomograms, Partin tables, and D’Amico classification for risk stratification.
Focal and Minimally Invasive Therapies
Focal therapy treats only the cancerous area, ideal for unilateral low-risk tumors. High-intensity focused ultrasound (HIFU) uses sound waves to destroy tissue. Cryotherapy freezes the tumor with minimal impact on erectile function, sometimes supported by Cialis Daily.
Photodynamic therapy activates light-sensitive drugs in the prostate. These options cut recurrence risk while aiding potency preservation. Best for patients rejecting whole-gland treatment.
Urologists assess via biopsy results and MRI prostate imaging. Post-treatment, track PSA nadir for success. These fit early stages without lymph node involvement.
What clinical trials are available for early-stage prostate cancer?
Active trials include NRG-GU010 comparing SBRT versus conventional RT with high accrual rates, PARTIQoL evaluating focal versus radical therapy for quality of life impacts, and ICECaP as a cryotherapy registry for localized prostate cancer.
These trials target patients with low-risk or intermediate-risk early-stage prostate cancer, often based on Gleason score, PSA levels, and stage T1 or T2 disease. Experts recommend discussing trial options with an oncologist or urologist to match personal risk stratification.
Participation can offer access to innovative treatments like stereotactic body radiation therapy or focal therapies while contributing to better outcomes in prostate health. Patients benefit from close monitoring and potential side effect management beyond standard care.
To find suitable trials, search ClinicalTrials.gov using terms like “prostate cancer stage T1 T2 recruiting”. The NCI matching service connects patients to relevant studies based on biopsy results and MRI findings.
Top 5 Ongoing Trials
| Trial | Phase | Intervention | Eligibility | Primary Endpoint | NCT# |
|---|---|---|---|---|---|
| NRG-GU010 | III | SBRT vs conventional RT | Low/intermediate-risk, stage T1-T2, Gleason 7 | Disease control | NCT04572649 |
| PARTIQoL | II | Focal HIFU vs radical prostatectomy | Localized low-risk, PSA <10, Gleason 3+3 | Quality of life at 2 years | NCT03991901 |
| ICECaP | Observational | Cryotherapy registry | Early-stage recurrent or primary, post-radiation | Safety and efficacy | NCT03682792 |
| PACE-B | III | SBRT vs surgery vs RT | Low/intermediate-risk, organ-confined | Functional outcomes | NCT01584258 |
| FLAME | III | IMRT boost with ADT | Intermediate-risk, T1-T2, Gleason 7 | Freedom from failure | NCT01168479 |
Review these trials for alignment with your risk group and preferences, such as preserving potency or continence. Consult NCCN or AUA guidelines alongside trial data for informed decisions on best treatment.
How to choose the right specialist for early-stage prostate cancer treatment?
Seek high-volume surgeons who perform more than 100 radical prostatectomies per year with low positive margin rates, along with radiation oncologists handling over 200 prostate cases annually, preferably at NCI-designated centers for better outcomes in early-stage prostate cancer.
Start by checking the specialist’s experience with your specific risk group, such as low-risk or intermediate-risk based on Gleason score and PSA levels. Ask about their approach to active surveillance versus treatments like robotic surgery or radiation therapy.
Experts recommend multidisciplinary teams that include urologists, radiation oncologists, and medical oncologists to discuss cases weekly. This setup ensures tailored plans for localized prostate cancer, balancing cure rates and quality of life.
Review surgeon report cards by asking direct questions about complication rates for erectile dysfunction and urinary incontinence. High-volume providers often achieve better continence recovery and potency preservation through nerve-sparing techniques.
Credentials checklist
Look for specialists who are fellowship-trained in urologic oncology or radiation oncology for early-stage prostate cancer. Membership in groups like ASTRO or AUA signals commitment to current guidelines such as NCCN or EAU standards.
Check if they regularly publish research on topics like robotic-assisted prostatectomy or intensity-modulated radiation therapy. This shows they stay updated on advances in brachytherapy and focal therapies like HIFU.
Ask about board certification and years treating T1 prostate cancer or T2 prostate cancer. For example, a urologist experienced with da Vinci robot may offer precise nerve-sparing for better side effect management.
Use this checklist during consultations: verify training, affiliations, and recent publications. It helps identify providers skilled in risk stratification using tools like nomograms, Partin tables, CAPRA score, or D’Amico classification with multiparametric MRI scan results.
Volume-outcome data
Research suggests high-volume surgeons performing over 50 prostate cases per year see fewer complications in radical prostatectomy or laparoscopic procedures. This matters for early-stage cases to minimize risks like positive margins or capsular penetration, while monitoring PSA levels.
For radiation oncologists, prioritize those with substantial experience in EBRT, IMRT, or SBRT for prostate cancer. Higher case volumes correlate with optimized plans that spare bowel and bladder function.
Inquire about annual prostate treatment numbers during your visit. High-volume centers often track outcomes for recurrence risk and post-treatment PSA monitoring, aiding decisions on active surveillance versus intervention.
Focus on providers at centers with proven volume data. This approach supports better quality of life after treatments like seed implants or proton therapy.
Multidisciplinary clinic
Choose clinics with weekly tumor board conferences involving urology, radiation, and medical oncology experts. They review biopsy results, MRI prostate imaging, and staging per NCCN guidelines, AUA guidelines, or EAU guidelines to recommend the best treatment for low-risk prostate cancer.
These teams weigh options like watchful waiting, hormone therapy, or robotic surgery based on tumor volume and DRE findings. Collaboration reduces overtreatment in early stages.
Ask if the clinic offers one-stop consultations for multiparametric MRI and bone scans. This streamlines planning for intermediate-risk cases, incorporating NCCN guidelines.
Benefits include personalized paths, such as combining ADT with radiation for higher-risk early disease. It ensures focus on five-year survival and long-term monitoring.
Surgeon report card questions
Prepare these key questions for urologists: What is your annual volume of radical prostatectomies? What rate of positive surgical margins do you achieve in organ-confined cases? Do you use LHRH agonists in neoadjuvant settings?
- How do you handle nerve-sparing surgery to preserve potency in low-volume disease? What outcomes do you see with Viagra or Cialis (Cialis Daily)?
- What is your patients’ typical continence recovery time after robotic prostatectomy? Do you recommend Kegel exercises for recovery?
- Can you share outcomes for Gleason 3+4 tumors regarding lymph node involvement?
Tailor questions to your profile, like PSA doubling time or Gleason score. Strong answers indicate expertise in early-stage treatment with low recurrence risk.
Frequently Asked Questions
What is the best treatment for prostate cancer in early stages?
The best treatment for prostate cancer in early stages depends on factors like the patient’s age, overall health, and cancer grade, but active surveillance is often recommended for low-risk cases to avoid unnecessary interventions while monitoring progression with PSA levels.
Is active surveillance the best treatment for prostate cancer in early stages for low-risk patients?
Yes, active surveillance is frequently considered the best treatment for prostate cancer in early stages when the cancer is low-risk, involving regular PSA tests, biopsies, and imaging to intervene only if the cancer shows signs of advancing.
What makes surgery a potential best treatment for prostate cancer in early stages?
Surgery, such as radical prostatectomy using the da Vinci robot, can be the best treatment for prostate cancer in early stages for healthier patients with intermediate-risk disease, as it removes the prostate gland entirely and offers a potential cure with high success rates.
How does radiation therapy serve as the best treatment for prostate cancer in early stages?
Radiation therapy, including external beam, IMRT, SBRT, or brachytherapy, is often the best treatment for prostate cancer in early stages for patients who prefer non-surgical options, precisely targeting and destroying cancer cells while preserving surrounding tissues.
When is hormone therapy combined with other options as the best treatment for prostate cancer in early stages?
Hormone therapy may be used alongside radiation as part of the best treatment for prostate cancer in early stages in intermediate- or higher-risk cases, reducing testosterone levels to slow cancer growth and improve outcomes.
Are focal therapies a viable best treatment for prostate cancer in early stages?
Focal therapies like HIFU or cryotherapy are emerging as potential best treatments for prostate cancer in early stages in select low-risk, localized tumors, targeting only the affected area to minimize side effects compared to whole-gland treatments.